Cancer Monitoring
The BEST tool for monitoring cancer in cats and dogs: Minimal sample, low cost, effective.
VDI’s Cancer Panels can be the most effective tool you have for monitoring cancer patients. The low-cost and minimal sample requirement makes it a clear choice for managing your patients.
Scroll down for Case Studies, Common Applications, and Report Interpretation
Monitoring Overview
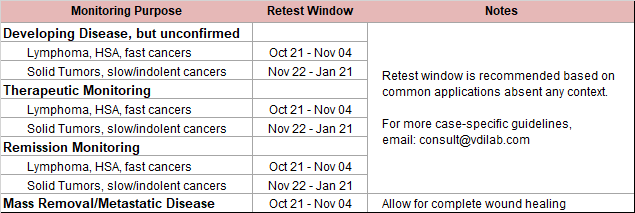
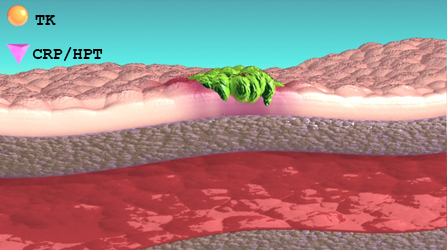
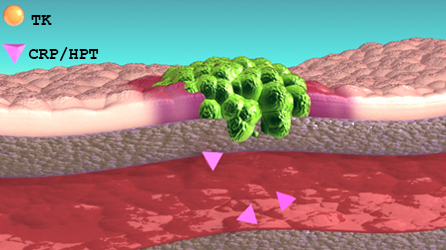
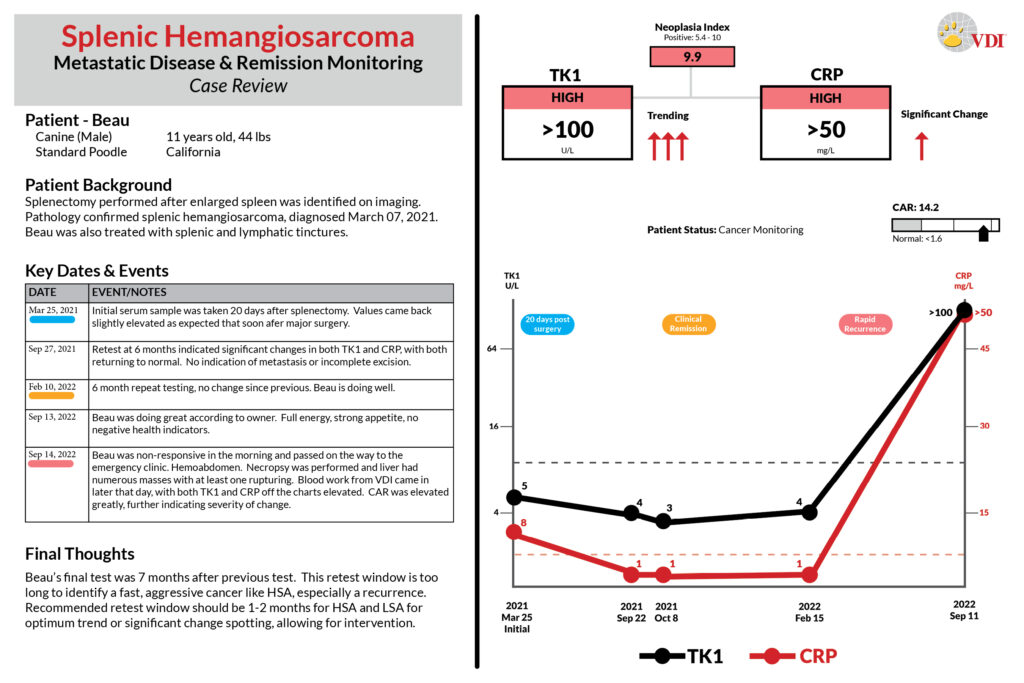
Thymidine Kinase, type 1 (TK1) is a DNA proliferation enzyme and is elevated in dividing cancer cells. During therapy (chemo/surgery) the source of TK1 is reduced/eliminated and serum TK1 (sTK1) levels will fall. Conversely, growing cancer cells during disease recurrence will increase sTK1 levels. Suspected undiagnosed patients can also be followed in the same manner. This makes the Cancer Panel an effective tool for patient monitoring.
When combined with an inflammatory marker (CRP/HPT), the change and relationship between both markers is indicative of disease activity.
Common Applications:
- Indolent cancer tracking
- Chemotherapy management
- Post mass-removal confirmation
- Metastatic disease
- Remission monitoring
Not recommended for:
- Cutaneous masses
- Mast Cell Tumors (stage I)
- Brain tumors
Interpreting the Report
Scroll down to learn more about what’s included with VDI cancer monitoring reports.
Reports Include:
- TK1 result
- C-Reactive Protein (dog) or Haptoglobin (cat) result
- Neoplasia Index (NI)® – for initial diagnosis if required
- Trending Arrows
- Significant Change Arrows
- Interpretive Comments
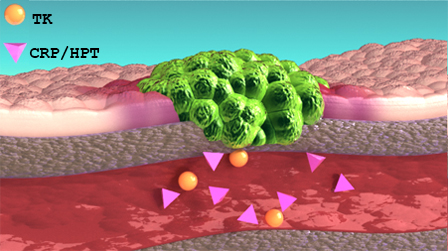
- Recommended Retest Window
- Contextual Review (must provide context on requisition form)
Sample Monitoring Reports:
REFERENCES – Body of Evidence
Boyé, Pierre, et al. “Evaluation of Serum Thymidine Kinase 1 Activity as a Biomarker for Treatment Effectiveness and Prediction of Relapse in Dogs With non‐Hodgkin Lymphoma.” Journal of Veterinary Internal Medicine, vol. 33, no. 4, Wiley, May 2019, pp. 1728–39. https://doi.org/10.1111/jvim.15513.
Euler, Henrik von, Anders B. Öhrvik, et al. “A Non-radiometric Method for Measuring Serum Thymidine Kinase Activity in Malignant Lymphoma in Dogs.” Research in Veterinary Science, vol. 80, no. 1, Feb. 2006, pp. 17–24. https://doi.org/10.1016/j.rvsc.2005.05.001.
Euler, Henrik von. “Monitoring Therapy in Canine Malignant Lymphoma and Leukemia With Serum Thymidine Kinase 1 Activity – Evaluation of a New, Fully Automated Non-radiometric Assay.” International Journal of Oncology, Jan. 1992, https://doi.org/10.3892/ijo_00000175.
Euler, Henrik von, Roland Einarsson, et al. “Serum Thymidine Kinase Activity in Dogs With Malignant Lymphoma: A Potent Marker for Prognosis and Monitoring the Disease.” Journal of Veterinary Internal Medicine, vol. 18, no. 5, Aug. 2004, p. 696. https://doi.org/10.1892/0891-6640(2004)18.
Grobman, M., et al. “Serum Thymidine Kinase 1, Canine-C-Reactive Protein, Haptoglobin, and Vitamin D Concentrations in Dogs With Immune-Mediated Hemolytic Anemia, Thrombocytopenia, and Polyarthropathy.” Journal of Veterinary Internal Medicine, vol. 31, no. 5, Wiley, Aug. 2017, pp. 1430–40. https://doi.org/10.1111/jvim.14787.
Larsdotter, S., et al. “Serum Thymidine Kinase Activity in Clinically Healthy and Diseased Horses: A Potential Marker for Lymphoma.” The Veterinary Journal, vol. 205, no. 2, Elsevier BV, Aug. 2015, pp. 313–16. https://doi.org/10.1016/j.tvjl.2015.01.019.
Meachem, Melissa D., et al. “A Comparative Proteomic Study of Plasma in Feline Pancreatitis and Pancreatic Carcinoma Using 2-dimensional Gel Electrophoresis to Identify Diagnostic Biomarkers: A Pilot Study.” Canadian Journal of Veterinary Research-revue Canadienne De Recherche Veterinaire, vol. 79, no. 3, June 2015, pp. 184–89.
NAKAMURA, Noriko, et al. “Plasma Thymidine Kinase Activity in Dogs With Lymphoma and Leukemia.” Journal of Veterinary Medical Science, vol. 59, no. 10, Japanese Society of Veterinary Science, 1997, pp. 957–60. https://doi.org/10.1292/jvms.59.957.
Selting, K. A., et al. “Serum Thymidine Kinase 1 and C‐reactive Protein as Biomarkers for Screening Clinically Healthy Dogs for Occult Disease.” Veterinary and Comparative Oncology, vol. 13, no. 4, Wiley, July 2013, pp. 373–84. https://doi.org/10.1111/vco.12052.
Selting, K.A., et al. “Thymidine Kinase Type 1 and C‐Reactive Protein Concentrations in Dogs With Spontaneously Occurring Cancer.” Journal of Veterinary Internal Medicine, vol. 30, no. 4, Wiley, May 2016, pp. 1159–66. https://doi.org/10.1111/jvim.13954.
Taylor, Samantha S., et al. “Serum Thymidine Kinase Activity in Clinically Healthy and Diseased Cats: A Potential Biomarker for Lymphoma.” Journal of Feline Medicine and Surgery, vol. 15, no. 2, SAGE Publications, Oct. 2012, pp. 142–47. https://doi.org/10.1177/1098612×12463928.
Thamm, D. H., et al. “Elevated Serum Thymidine Kinase Activity in Canine Splenic Hemangiosarcoma*.” Veterinary and Comparative Oncology, vol. 10, no. 4, Wiley, Oct. 2011, pp. 292–302. https://doi.org/10.1111/j.1476-5829.2011.00298.x.
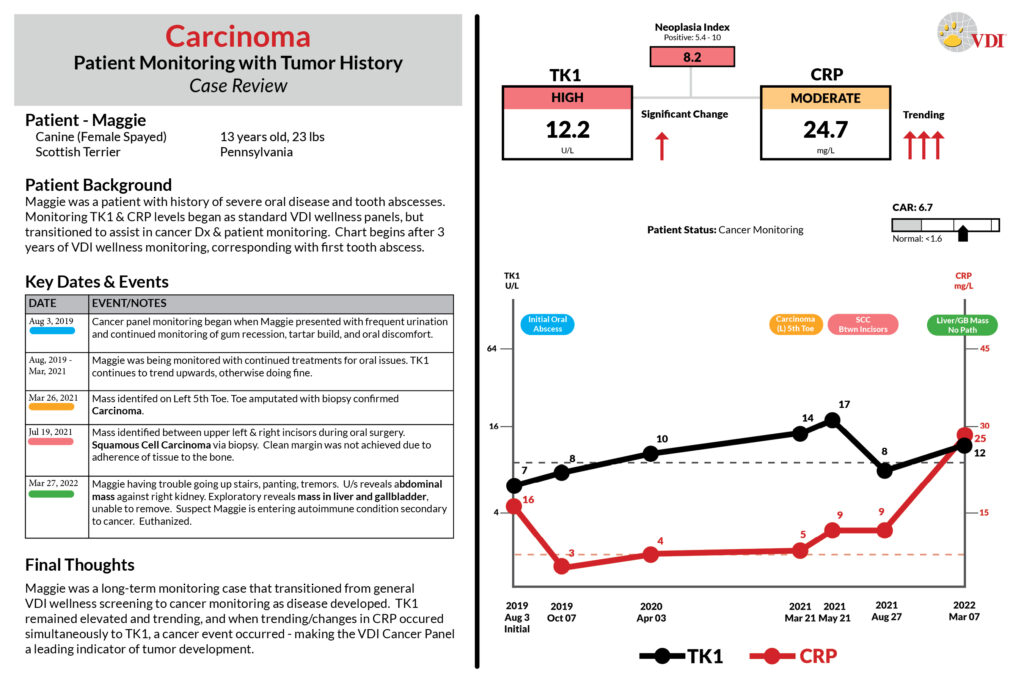
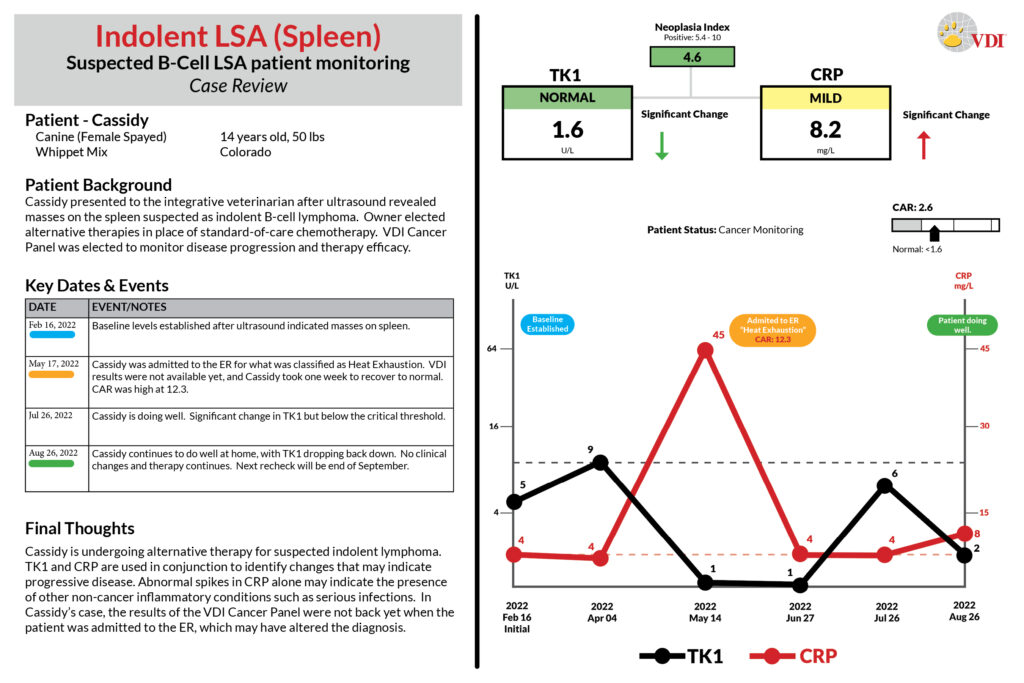
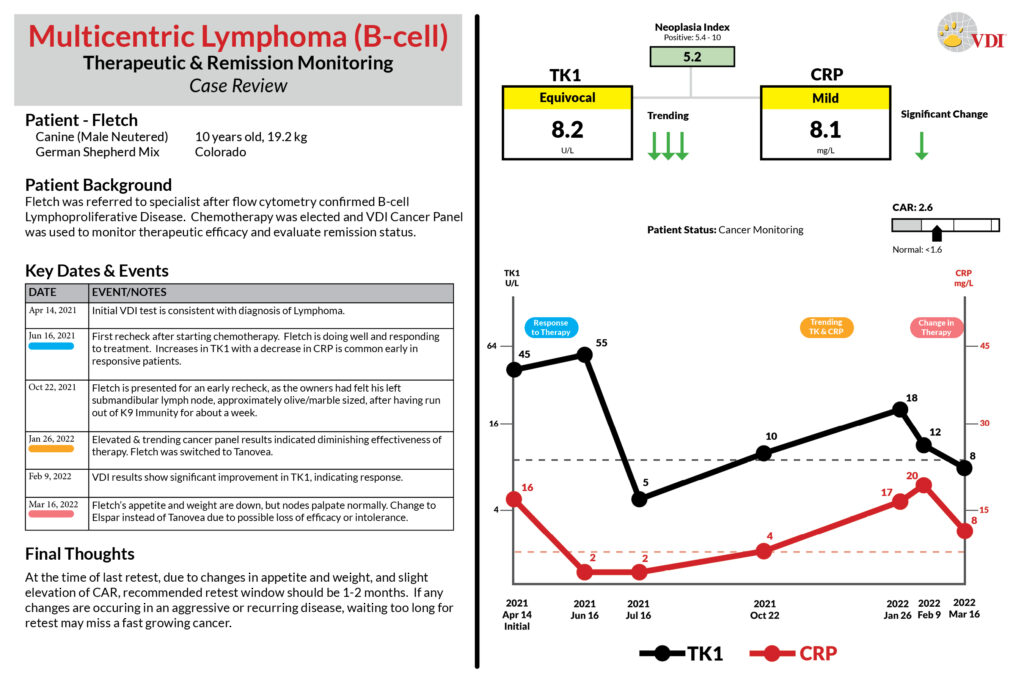
Case Studies
Scroll the case studies below, or click the links:
Interpreting the Results
VDI Cancer Panel monitoring reports come with a number of important indicators. Reports come with general comments reflecting the information in the tables below, but each cancer panel report still receives a contextual review by the lab for additional comments/interpretation as needed.
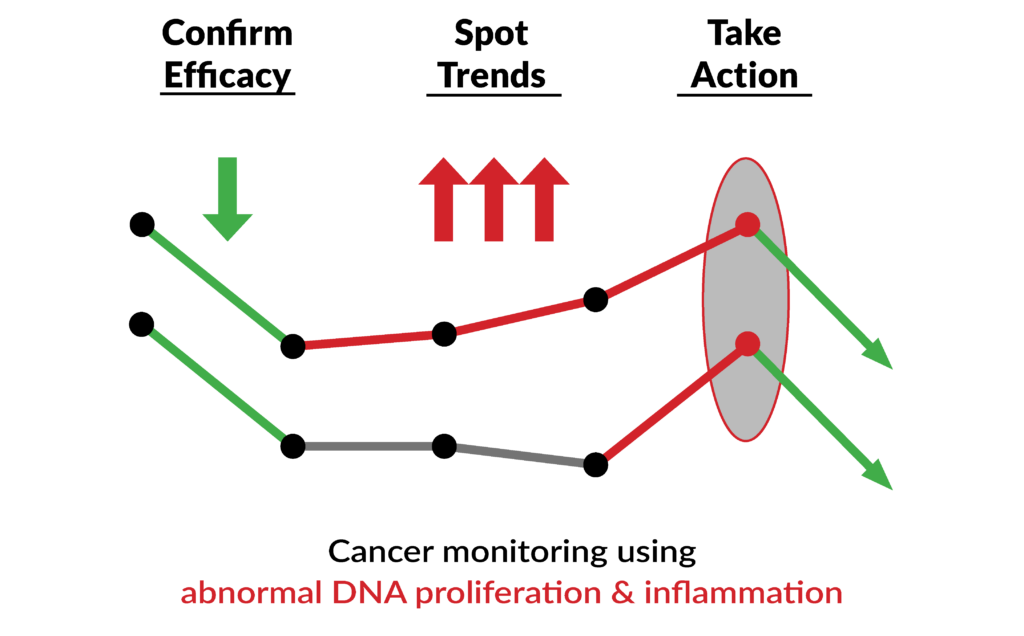
Significant Change
A change of 40% or more from prior. Studies show this level of change can precede cancer recurrence.
| What does it signify? | What does it mean? | |
|---|---|---|
 | significant reduction or improvement of at least 40% | indicates therapy is effective in reducing or eliminating the tumor or inflammation |
 | significant increase in TK1 however below the 9U/L threshold | indicates the level of change is worth watching – changes in clinical status of the patient is important |
 | significant increase in the biomarker above critical thresholds | studies show this level of change in TK1 often precedes cancer recurrence |
Trending
2 or more data points in the same direction. Short-term trending events are the most valuable in confirming cancer recurrence.
| What does it signify? | What does it mean? | |
|---|---|---|
 | significant reduction or improvement of at least 40% | indicates therapy is effective in reducing or eliminating the tumor or inflammation |
 | significant increase in TK1 however below the 9U/L threshold | indicates the level of change is worth watching – changes in clinical status of the patient is important |
 | significant increase in the biomarker above critical thresholds | studies show this level of change in TK1 often precedes cancer recurrence |
Retest Window
With VDI Cancer Panel monitoring reports, you may also receive a retest window recommendation. These recommendations are to help ensure you are rechecking TK1 and CRP/HPT levels in a timeframe that will have the most impact for patient evaluation. These windows are automatically calculated based on various factors and when the current sample was collected.